Introduction
Caesalpinioideae, which includes the nested mimosoid clade (former subfamily Mimosoideae), comprises 163 genera and c. 4680 species, with c. 75% of the species in the mimosoid clade. Recent phylogenomic work provides a robust phylogenomic backbone for a new tribal classification of Caesalpinioideae, which is forthcoming soon.
Caesalpinioideae date to the late Paleocene when the subfamily is known from fossil bipinnate leaves from Colombia. These fossils indicate that Caesalpinioideae were already an abundant element in the earliest Neotropical rain forests in the Paleocene and started to diversify around 58 million years ago. Caesalpinioideae have thus diversified throughout the Cenozoic and now comprise diverse, abundant, and sometimes dominant elements across all major lowland tropical biomes, including tropical rain forests, savannas and seasonally dry tropical forests.
The Caesalpinioideae include some of the largest genera in the legume family, including Acacia, with > 1,000 species concentrated in arid parts of Australia, and Mimosa with 615 species mostly in the Neotropics. Other species-rich genera include Inga with c. 300 species restricted to the Neotropics almost entirely in rainforests, the pantropical Chamaecrista (361 species) and Senna (287 species), whose highest diversity is found in the Neotropics, as well as Vachellia (164 species) and Senegalia (219 species), two pantropical genera concentrated in drier environments, within which the iconic umbrella-crown trees of savannas, especially in Africa, are found.
Key Features
Caesalpinioideae are almost entirely woody perennials, but within those bounds they are extremely diverse in stature and habit – including lianas, trees of all sizes up to rain forest canopy emergents (e.g., Cedrelinga, Dinizia), shrubs and functionally herbaceous geoxyles. A handful of herbaceous aquatics are found in the genus Neptunia.
Caesalpinioideae is the only legume subfamily which has bipinnate leaves, which are prevalent but nor universal across the subfamily. Some genera have species with pinnate leaves, and leaves modified into phyllodes occur in the majority of species of the large genus Acacia and a few species in other genera including Senna and Mimosa. There is considerable quantitative variation in leaf dimensions and dividedness of leaves and especially the bipinnate leaf, from the massive leaves of e.g. Schizolobium (> 1m long), to highly reduced leaves and even aphyllous species (e.g. Prosopis kuntzei).

Nyctinastic leaf movements are common across all legumes, including Caesalpinioideae, but seismonasty, i.e. leaf movements that are prompted by touch, are only known within subfamily Caesalpinioideae in the genera Neptunia and Mimosa. The latter was referred to as Sensitivae Censitae by Rupert Barneby in his monograph of New World Mimosa, and Mimosa pudica has come to be grown as a pet house plant.
Extrafloral nectaries (EFNs) are usually present, and often conspicuous and abundant, on the petiole and/or the primary or secondary leaf rachises between pinnae or leaflet pairs in the majority of Caesalpinioideae. Indeed, it is within subfamily Caesalpinioideae, and especially the mimosoid clade, where the greatest concentration, diversity and abundance of EFN-possessing taxa within the legumes occur. Within the mimosoid clade, 90% of the 100 genera that are currently recognised possess EFNs.
Within Caesalpinioideae, some of the best-studied legume myrmecophytes, i.e. true ant plants with domatia, are found, including the emblematic swollen-thorn ant ‘acacias’ in the genus Vachellia in Africa and the Neotropics and in the genus Tachigali, albeit, in the latter case, the mutualism lacks EFNs. The Neotropical Vachellia myrmecophyte lineage with 12 species is often cited as one of the best-studied examples of co-evolution involving an obligate symbiotic mutualism between the Pseudomyrmex ferrugineus group of c. 10 species of ants that all nest exclusively in the swollen stipular spine domatia of Vachellia species. In return, the nectar from the multiple conspicuous EFNs on the petiole and leaf rachis, as well as specialised beltian food bodies on the tips of the leaflets, are specific for the resident ants.
Caesalpinioideae are commonly, but far from universally, armed with prickles or nodal / infranodal spines. Within the mimosoid clade, armature is restricted to the core mimosoid clade. Armature is highly variable, even among congeneric species. For example, within the small genus Parkinsonia trees can be completely unarmed, have stipular spines or spinescent leaf rachises.
Across the subfamily, inflorescences and flowers are also morphologically highly variable. Flowers are generally radially symmetrical in the mimosoid clade, but in some clades of Caesalpinioideae they are bilaterally symmetrical or asymmetrical. In the mimosoid clade, there is a progressive trend to reduced size of the perianth parts and showy exserted stamen filaments, with inflorescences rather than individual flowers taking over as the unit of pollinator attraction. The mimosoid clade has characteristic globose capitulate or spicate and frequently heteromorphic inflorescences often with sterile flowers which sometimes become showy staminodia. While a large majority of Caesalpinioideae flowers are bee pollinated, specialized bat and moth pollinated flowers are also common, especially in some largely night-flowering genera, such as Calliandra and Inga, in the ingoid clade. Caesalpinioideae is the only subfamily of legumes where pollen is arranged in polyads. These are extremely variable across and sometimes within genera, with pollen in monads, tetrads, bitetrads and polyads.
Caesalpinioideae are prominent, diverse and sometimes dominant in savannas. Many species show striking fire adaptations, including thick corky bark and functionally herbaceous geoxyle habit with large underground lignotubers or xylopodia.
One of the hallmarks of Caesalpinioideae, like many plant groups, are repeated morphological and ecological convergences whereby similar leaf, flower and fruit morphologies have been reinvented multiple times across the phylogeny. This is especially notable among fruits of the mimosoid clade which have proved misleading for generic delimitation, and which reflect selection pressures for different seed dispersal syndromes, including passive and explosive dehiscence, water, wind, large herbivores, ants, and birds.
Distribution and Ecology
The vast majority of Caesalpinioideae diversity is tropical, but a small fraction of species extend to the warm temperate zone, a subset of which are frost tolerant (Gleditsia, Gymnocladus, some Desmanthus). Caesalpinioideae are infrequent above 2500 m in the tropics and are largely absent from mid- and high-elevation tropical montane forests. Generic diversity is highest in the Neotropics, and there are important centres of high species diversity in Mexico / Central America, lowland South America, Africa, Madagascar, parts of SE Asia and Australia.
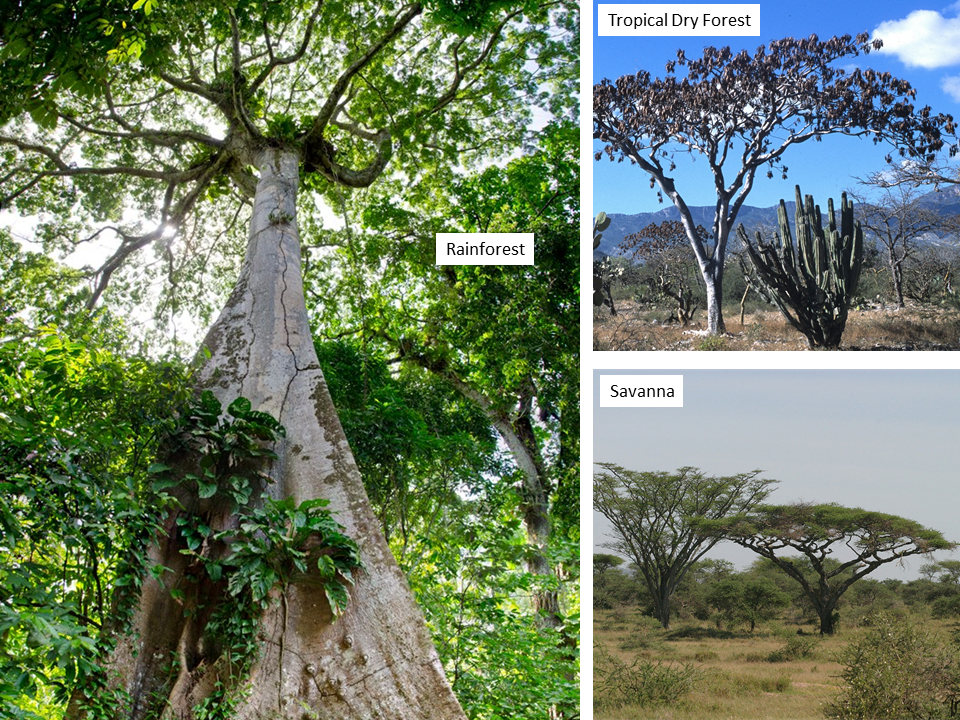 Caesalpinioideae are abundant and diverse woody elements across the lowland tropics in rain forests,
seasonally dry vegetation of the succulent biome and savannas
Caesalpinioideae are abundant and diverse woody elements across the lowland tropics in rain forests,
seasonally dry vegetation of the succulent biome and savannas
Formal Botanical Description
As published in LPWG (2017), Taxon 66: 44-77, doi.org/10.12705/661.3
Subfam. Caesalpinioideae DC., Prodr. 2: 473. 1825, emend. LPWG. Type: Caesalpinia L. = Mimosoideae DC., Prodr. 2: 424. 1825. Type Genus: Mimosa L. = Cassioideae Burmeist., Handb. Naturgesch.: 319. 1837.
Type Genus: Cassia L.
Trees, shrubs, lianas, suffruticose or functionally herbaceous, occasionally aquatic, either unarmed or commonly armed with prickles or nodal or infranodal spines; specialised extrafloral nectaries often present on the petiole and/or on the primary and secondary rachises, usually between pinnae or leaflet pairs, more rarely stipular or bracteal (Senna Mill., Macrosamanea Britton & Rose ex Britton & Killip and some Archidendron F. Muell.). Stipules in lateral position and free or absent. Leaves usually pulvinate, commonly bipinnate, otherwise pinnate (sometimes both types on the same plant in Arcoa Urb., Cenostigma Tul., Gleditsia L., Stuhlmannia Taub., rarely in Ceratonia L. and Moldenhawera Schrad.) and then mostly paripinnate, rarely imparipinnate, less often bifoliolate, modified into phyllodes or lacking, arrangement of the pinnae and leaflets mostly opposite, rarely alternate; stipels rare and not to be confused with the more commonly present paraphyllidia (reduced basal leaflet pair on the pinnae). Inflorescences globose, spicate, paniculate, racemose or in fascicles; bracteoles commonly absent or small. Flowers usually bisexual, rarely unisexual (Ceratonia, Gleditsia and Gymnocladus Lam., species dioecious or monoecious), or bisexual flowers combined with unisexual and/or sterile flowers in heteromorphic inflorescences (Mimosoid clade), radially, less frequently bilaterally symmetrical or asymmetric, hypanthium lacking or cupular, rarely tubular; sepals (3–) 5 (–6), free or fused; petals (3–) 5 (–6), free or fused (either one or both whorls sometimes lacking), aestivation valvate (Mimosoid clade) or imbricate and then the adaxial petal innermost; stamens commonly diplostemonous or haplostemonous, sometimes reduced to 3, 4 or 5 (in some Mimosa spp.), frequently many (100+ in some Mimosoid clade), free or fused, sometimes heteromorphic, some or all sometimes modified or staminodial, anthers basifixed or dorsifixed, often with a stipitate or sessile apical gland, dehiscing via long longitudinal slits or apical or basal poricidal slits or pores; pollen in tricolporate monads, or commonly in tetrads, bitetrads or polyads (most Mimosoid clade); gynoecium uni- or rarely polycarpellate, 1-many ovulate. Fruit a thin-valved, 1-many seeded pod, dehiscent along one or both sutures, also often a lomentum, lomentaceous, a craspedium or thick and woody and then indehiscent or explosively dehiscent, often curved or spirally coiled. Seeds usually with an open or closed pleurogram on both faces, sometimes with a fleshy aril (Pithecellobium Mart. and some Acacia Mill.) or sarcotesta (Inga Mill.), sometimes winged; hilum usually apical, lens usually inconspicuous; embryo straight. Vestured pits present in secondary xylem. Root nodules variably present and indeterminate (prevalent in Mimosoid clade). 2n mostly 24, 26, 28, but also reported 2n = 14, 16, 24, 26, 28, 52, 54, 56. Non-protein amino acids frequently reported, for example mimosine, albizine (Mimosoid clade), djenkolic acid, pipecolic acid and its derivatives; coumarins, cyanogenic glucosides, phenylethylamines, tryptamines, and β-carboline alkaloids also reported.
To learn more
Guinet, P., 1981. Mimosoideae: the characters of their pollen grains. Mimosoideae: the characters of their pollen grains.,In Polhill, R.M. & Raven, P.H. (Eds.) Advances in Legume Systematics Part 2, pp.835-857.
Hughes CE, Queiroz LP de, Lewis GP (Editors), 2022. Advances in Legume Systematics 14. Classification of Caesalpinioideae Part 1: new generic delimitations. PhytoKeys, 205, pp.1-470.
Koenen, E.J., Kidner, C., de Souza, É.R., Simon, M.F., Iganci, J.R., Nicholls, J.A., Brown, G.K., de Queiroz, L.P., Luckow, M., Lewis, G.P. and Pennington, R.T., 2020. Hybrid capture of 964 nuclear genes resolves evolutionary relationships in the mimosoid legumes and reveals the polytomous origins of a large pantropical radiation. American Journal of Botany, 107(12), pp.1710-1735.
The Legume Phylogeny Working Group (LPWG), 2017. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon, 66(1), pp.44-77.
Luckow, M., Miller, J.T., Murphy, D.J. and Livshultz, T., 2003. A phylogenetic analysis of the Mimosoideae (Leguminosae) based on chloroplast DNA sequence data. Advances in legume systematics, part, 10, pp.197-220.
Manzanilla, V. and Bruneau, A., 2012. Phylogeny reconstruction in the Caesalpinieae grade (Leguminosae) based on duplicated copies of the sucrose synthase gene and plastid markers. Molecular Phylogenetics and Evolution, 65(1), pp.149-162.
List of genera
Below is an alphabetical list of all genera accepted by the LPWG with links out to the taxonomic pages on our portal, GBIF and World Checklist of Vascular Plants (Kew). Over time this list will be updated to reflect the evolving taxonomy.
Please see the Species List and Synonyms and Legume Taxonomy Working Group pages for more taxonomic information. The current taxonomy is accessible by Browse or Advanced Search.
Genera indicated with an asterisk (*) are members of the mimosoid clade.
| Genus | Data Source | ||
|---|---|---|---|
| *Abarema Pittier | Legume Data Portal | GBIF | POWO |
| *Acacia Mill. | Legume Data Portal | GBIF | POWO |
| *Acaciella Britton & Rose | Legume Data Portal | GBIF | POWO |
| Acrocarpus Wight & Arn. | Legume Data Portal | GBIF | POWO |
| *Adenanthera L. | Legume Data Portal | GBIF | POWO |
| *Adenopodia C.Presl | Legume Data Portal | GBIF | POWO |
| *Afrocalliandra E.R.Souza & L.P.Queiroz | Legume Data Portal | GBIF | POWO |
| *Alantsilodendron Villiers | Legume Data Portal | GBIF | POWO |
| *Albizia Durazz. | Legume Data Portal | GBIF | POWO |
| *Amblygonocarpus Harms | Legume Data Portal | GBIF | POWO |
| *Anadenanthera Speg. | Legume Data Portal | GBIF | POWO |
| *Anonychium (Benth.) Schweinf. | [Legume Data Portal] | [GBIF] | [POWO] |
| Arapatiella Rizzini & A.Mattos | Legume Data Portal | GBIF | POWO |
| *Archidendron F.Muell. | Legume Data Portal | GBIF | POWO |
| *Archidendropsis I.C.Nielsen | Legume Data Portal | GBIF | POWO |
| Arcoa Urb. | Legume Data Portal | GBIF | POWO |
| Arquita Gagnon, G.P.Lewis & C.E.Hughes | Legume Data Portal | GBIF | POWO |
| *Aubrevillea Pellegr. | Legume Data Portal | GBIF | POWO |
| *Balizia Barneby & J.W.Grimes | Legume Data Portal | GBIF | POWO |
| Balsamocarpon Clos | Legume Data Portal | GBIF | POWO |
| Batesia Spruce ex Benth. & Hook.f. | Legume Data Portal | GBIF | POWO |
| Biancaea Tod. | Legume Data Portal | GBIF | POWO |
| *Blanchetiodendron Barneby & J.W.Grimes | Legume Data Portal | GBIF | POWO |
| *Boliviadendron E.R.Souza & C.E.Hughes | [Legume Data Portal] | [GBIF] | [POWO] |
| Burkea Benth. | Legume Data Portal | GBIF | POWO |
| Bussea Harms | Legume Data Portal | GBIF | POWO |
| Caesalpinia Plum ex L. | Legume Data Portal | GBIF | POWO |
| *Calliandra Benth. | Legume Data Portal | GBIF | POWO |
| *Calliandropsis H.M.Hern. & P.Guinet | Legume Data Portal | GBIF | POWO |
| *Calpocalyx Harms | Legume Data Portal | GBIF | POWO |
| Campsiandra Benth. | Legume Data Portal | GBIF | POWO |
| Cassia L. | Legume Data Portal | GBIF | POWO |
| *Cathormion (Benth.) Hassk. NO LONGER RECOGNISED | Legume Data Portal | GBIF | POWO |
| *Cedrelinga Ducke | Legume Data Portal | GBIF | POWO |
| Cenostigma Tul. | Legume Data Portal | GBIF | POWO |
| Ceratonia L. | Legume Data Portal | GBIF | POWO |
| Chamaecrista Moench | Legume Data Portal | GBIF | POWO |
| *Chidlowia Hoyle | Legume Data Portal | GBIF | POWO |
| *Chloroleucon (Benth.) Britton & Rose | Legume Data Portal | GBIF | POWO |
| *Cojoba Britton & Rose | Legume Data Portal | GBIF | POWO |
| Colvillea Bojer ex Hook. | Legume Data Portal | GBIF | POWO |
| Conzattia Rose | Legume Data Portal | GBIF | POWO |
| Cordeauxia Hemsl. | Legume Data Portal | GBIF | POWO |
| Coulteria Kunth | Legume Data Portal | GBIF | POWO |
| *Cylicodiscus Harms | Legume Data Portal | GBIF | POWO |
| Delonix Raf. | Legume Data Portal | GBIF | POWO |
| Denisophytum R.Vig. | Legume Data Portal | GBIF | POWO |
| *Desmanthus Willd. | Legume Data Portal | GBIF | POWO |
| *Dichrostachys (DC.) Wight & Arn. | Legume Data Portal | GBIF | POWO |
| Dimorphandra Schott | Legume Data Portal | GBIF | POWO |
| Dinizia Ducke | Legume Data Portal | GBIF | POWO |
| Diptychandra Tul. | Legume Data Portal | GBIF | POWO |
| *Ebenopsis Britton & Rose | Legume Data Portal | GBIF | POWO |
| *Elephantorrhiza Benth. NO LONGER RECOGNISED | Legume Data Portal | GBIF | POWO |
| *Entada Adans. | Legume Data Portal | GBIF | POWO |
| *Enterolobium Mart. | Legume Data Portal | GBIF | POWO |
| Erythrophleum Afzel. ex G.Don | Legume Data Portal | GBIF | POWO |
| Erythrostemon Klotzsch | Legume Data Portal | GBIF | POWO |
| *Faidherbia A.Chev. | Legume Data Portal | GBIF | POWO |
| *Falcataria (I.C.Nielsen) Barneby & J.W.Grimes | Legume Data Portal | GBIF | POWO |
| *Fillaeopsis Harms | Legume Data Portal | GBIF | POWO |
| *Gagnebina Neck. ex DC. | Legume Data Portal | GBIF | POWO |
| Gelrebia Gagnon & G.P.Lewis | Legume Data Portal | GBIF | POWO |
| Gleditsia L. | Legume Data Portal | GBIF | POWO |
| *Gretheria R.Duno & Torke | [Legume Data Portal] | [GBIF] | [POWO] |
| Guilandina L. | Legume Data Portal | GBIF | POWO |
| *Gwilymia A.Lima, Paula-Souza & Scalon | [Legume Data Portal] | [GBIF] | [POWO] |
| Gymnocladus Lam. | Legume Data Portal | GBIF | POWO |
| Haematoxylum L. | Legume Data Portal | GBIF | POWO |
| *Havardia Small | Legume Data Portal | GBIF | POWO |
| *Heliodendron Gill.K.Br. & Bayly | [Legume Data Portal] | [GBIF] | [POWO] |
| Hererolandia Gagnon & G.P.Lewis | Legume Data Portal | GBIF | POWO |
| *Hesperalbizia Barneby & J.W.Grimes | [Legume Data Portal] | GBIF | [POWO] |
| Heteroflorum M.Sousa | Legume Data Portal | GBIF | POWO |
| Hoffmannseggia Cav. | Legume Data Portal | GBIF | POWO |
| Hultholia Gagnon & G.P.Lewis | Legume Data Portal | GBIF | POWO |
| *Hydrochorea Barneby & J.W.Grimes | Legume Data Portal | GBIF | POWO |
| *Indopiptadenia Brenan | Legume Data Portal | GBIF | POWO |
| *Inga Mill. | Legume Data Portal | GBIF | POWO |
| Jacqueshuberia Ducke | Legume Data Portal | GBIF | POWO |
| *Jupunba Britton & Rose | Legume Data Portal | GBIF | POWO |
| *Kanaloa Lorence & K.R.Wood | Legume Data Portal | GBIF | POWO |
| *Lachesiodendron P.G.Ribeiro, L.P.Queiroz & Luckow | Legume Data Portal | GBIF | POWO |
| Lemurodendron Villiers & P.Guinet | Legume Data Portal | GBIF | POWO |
| *Leucaena Benth. | Legume Data Portal | GBIF | POWO |
| *Leucochloron Barneby & J.W.Grimes | Legume Data Portal | GBIF | POWO |
| Libidibia (DC.) Schltdl. | Legume Data Portal | GBIF | POWO |
| Lophocarpinia Burkart | Legume Data Portal | GBIF | POWO |
| *Lysiloma Benth. | Legume Data Portal | GBIF | POWO |
| *Macrosamanea Britton & Rose ex Britton & Killip | Legume Data Portal | GBIF | POWO |
| *Mariosousa Seigler & Ebinger | Legume Data Portal | GBIF | POWO |
| *Marlimorimia L.P.Queiroz, L.M.Borges, Marc.F.Simon & P.G.Ribeiro | [Legume Data Portal] | [GBIF] | [POWO] |
| Melanoxylum Schott | GBIF | ||
| *Mezcala C.E.Hughes & J.L.Contr. | [Legume Data Portal] | [GBIF] | [POWO] |
| Mezoneuron Desf. | Legume Data Portal | GBIF | POWO |
| *Microlobius C.Presl | Legume Data Portal | GBIF | POWO |
| *Mimosa L. | Legume Data Portal | GBIF | POWO |
| *Mimozyganthus Burkart | GBIF | POWO | |
| Moldenhawera Schrad. | Legume Data Portal | GBIF | POWO |
| Mora R.H.Schomb. ex Benth. | Legume Data Portal | GBIF | POWO |
| Moullava Adans. | Legume Data Portal | GBIF | POWO |
| *Naiadendron A.G.Lima, Paula-Souza & Scalon | [Legume Data Portal] | [GBIF] | [POWO] |
| *Neltuma Raf. | [Legume Data Portal] | GBIF | [POWO] |
| *Neptunia Lour. | Legume Data Portal | GBIF | POWO |
| *Newtonia Baill. | Legume Data Portal | GBIF | POWO |
| *Osodendron E.J.M.Koenen | [Legume Data Portal] | [GBIF] | [POWO] |
| Pachyelasma Harms | Legume Data Portal | GBIF | POWO |
| *Painteria Britton & Rose | Legume Data Portal | GBIF | POWO |
| *Parapiptadenia Brenan | Legume Data Portal | GBIF | POWO |
| *Pararchidendron I.C.Nielsen | Legume Data Portal | GBIF | POWO |
| *Parasenegalia Siegler & Ebinger | Legume Data Portal | GBIF | POWO |
| *Paraserianthes I.C.Nielsen | Legume Data Portal | GBIF | POWO |
| *Parkia R.Br. | Legume Data Portal | GBIF | POWO |
| Parkinsonia Plum ex L. | Legume Data Portal | GBIF | POWO |
| Paubrasilia Gagnon, H.C.Lima & G.P.Lewis | Legume Data Portal | GBIF | POWO |
| Peltophorum (Vogel) Benth. | Legume Data Portal | GBIF | POWO |
| *Pentaclethra Benth. | Legume Data Portal | GBIF | POWO |
| *Piptadenia Benth. | Legume Data Portal | GBIF | POWO |
| *Piptadeniastrum Brenan | Legume Data Portal | GBIF | POWO |
| *Piptadeniopsis Burkart | Legume Data Portal | GBIF | POWO |
| *Pithecellobium Mart. | Legume Data Portal | GBIF | POWO |
| *Pityrocarpa (Benth. & Hook.f.) Britton & Rose | Legume Data Portal | GBIF | POWO |
| *Plathymenia Benth. | Legume Data Portal | GBIF | POWO |
| Pomaria Cav. | Legume Data Portal | GBIF | POWO |
| *Prosopidastrum Burkart | Legume Data Portal | GBIF | POWO |
| *Prosopis L. | GBIF | POWO | |
| *Pseudalbizzia Britton & Rose | [Legume Data Portal] | GBIF | [POWO] |
| *Pseudopiptadenia Rauschert NO LONGER RECOGNISED | Legume Data Portal | GBIF | POWO |
| *Pseudoprosopis Harms | Legume Data Portal | GBIF | POWO |
| *Pseudosamanea Harms | Legume Data Portal | GBIF | POWO |
| *Pseudosenegalia Siegler & Ebinger | Legume Data Portal | GBIF | POWO |
| Pterogyne Tul. | Legume Data Portal | GBIF | POWO |
| Pterolobium R.Br. ex Wight & Arn. | Legume Data Portal | GBIF | POWO |
| *Punjuba Britton & Rose | Legume Data Portal | GBIF | POWO |
| Recordoxylon Ducke | Legume Data Portal | GBIF | POWO |
| *Ricoa Duno & Torke | [Legume Data Portal] | [GBIF] | [POWO] |
| *Robrichia (Barneby & J.W.Grimes) A.R.M.Luz & E.R.Souza | [Legume Data Portal] | [GBIF] | [POWO] |
| *Samanea (Benth.) Merr. | Legume Data Portal | GBIF | POWO |
| *Sanjappa E.R.Souza & Krishnaraj | Legume Data Portal | GBIF | POWO |
| Schizolobium Vogel | Legume Data Portal | GBIF | POWO |
| *Schleinitzia Warb. | Legume Data Portal | GBIF | POWO |
| *Senegalia Raf. | Legume Data Portal | GBIF | POWO |
| Senna Mill. | Legume Data Portal | GBIF | POWO |
| *Serianthes Benth. | Legume Data Portal | GBIF | POWO |
| *Sphinga Barneby & J.W.Grimes | Legume Data Portal | GBIF | POWO |
| Stachyothyrsus Harms | Legume Data Portal | GBIF | POWO |
| Stenodrepanum Harms | Legume Data Portal | GBIF | POWO |
| *Strombocarpa Engelm. & A.Gray | [Legume Data Portal] | [GBIF] | [POWO] |
| *Stryphnodendron Mart. | Legume Data Portal | GBIF | POWO |
| Stuhlmannia Taub. | Legume Data Portal | GBIF | POWO |
| *Sympetalandra Stapf | Legume Data Portal | GBIF | POWO |
| Tachigali Aubl. | Legume Data Portal | GBIF | POWO |
| Tara Molina | Legume Data Portal | GBIF | POWO |
| *Tetrapleura Benth. | Legume Data Portal | GBIF | POWO |
| Tetrapterocarpon Humbert | Legume Data Portal | GBIF | POWO |
| *Thailentadopsis Kosterm. | Legume Data Portal | GBIF | POWO |
| Ticanto Adans. | [Legume Data Portal] | GBIF | [POWO] |
| Umtiza Sim | Legume Data Portal | GBIF | POWO |
| *Vachellia Wight & Arn. | Legume Data Portal | GBIF | POWO |
| *Viguieranthus Villiers | Legume Data Portal | GBIF | POWO |
| Vouacapoua Aubl. | Legume Data Portal | GBIF | POWO |
| *Wallaceodendron Koord. | Legume Data Portal | GBIF | POWO |
| *Xerocladia Harv. | Legume Data Portal | GBIF | POWO |
| *Xylia Benth. | Legume Data Portal | GBIF | POWO |
| *Zapoteca H.M.Hern. | Legume Data Portal | GBIF | POWO |
| Zuccagnia Cav. | Legume Data Portal | GBIF | POWO |
| *Zygia P.Browne | Legume Data Portal | GBIF | POWO |
